From Teknor Apex come new TPEs for overmoulding that are said to exhibit excellent adhesion to engineering plastic substrates while meeting the stringent requirements for use in medical devices. The Medalist MD-30000 series bond to PC, ABS, PC/ABS, CoPe, PET, PBT, ASA, SAN, PMMA, POM, PA, and PS. Each compound in the series is chemically modified for adhesion to specific substrates. Processable in either multi-shot or insert moulding, the compounds are for use in wearable devices, ‘soft-touch’ or cosmetic grips for device housings, handheld devices, instruments, and seals and gaskets.
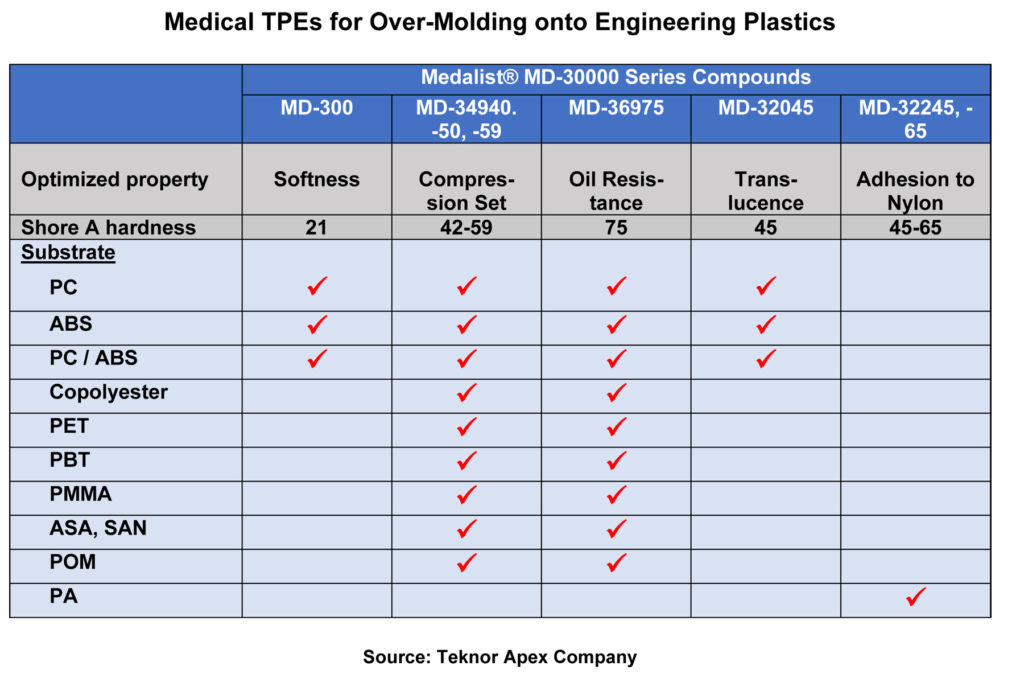
The new series includes grades with enhanced softness, translucence, low compression set for effective sealing properties, and resistance to lotions and other chemicals encountered by wearable devices. Unlike liquid silicones, overmoulded TPEs do not require the use of a primer, have a shorter cycle time due to avoidance of a curing process, and can be processed on conventional injection moulding equipment.
Three of the Medalist MD-30000 Series compounds were introduced earlier this year after successful completion of a joint project with Covestro LLC, in which the materials were overmoulded onto medical grades of PC and PC alloys and tested for adhesion, processability, and chemical resistance.
“Overmoulding Medalist TPEs onto rigid substrates enhances the ergonomic and aesthetic properties of medical devices, adds new functionalities such as moulded-in seals and cushions, and provides the design freedom and cost savings associated with parts consolidation,” said Ross van Royen, senior market manager for Teknor Apex. “The Medalist MD-30000 series is the latest addition to a large portfolio of TPEs developed by Teknor Apex for overmoulding applications in medical devices as well as in the consumer product and automotive industries.”
Medalist MD-30000 Series compounds are offered worldwide. Custom formulations are available. As medical-grade TPEs, they are subject to strict formulation controls, are made only with FDA-listed food-grade ingredients, are ISO 10993-5 compliant for biocompatibility, and are compliant with CONEG, RoHS, and California Proposition 65 requirements. The compounds are produced in multiple ISO-13485-certified facilities, thus ensuring security of supply.
