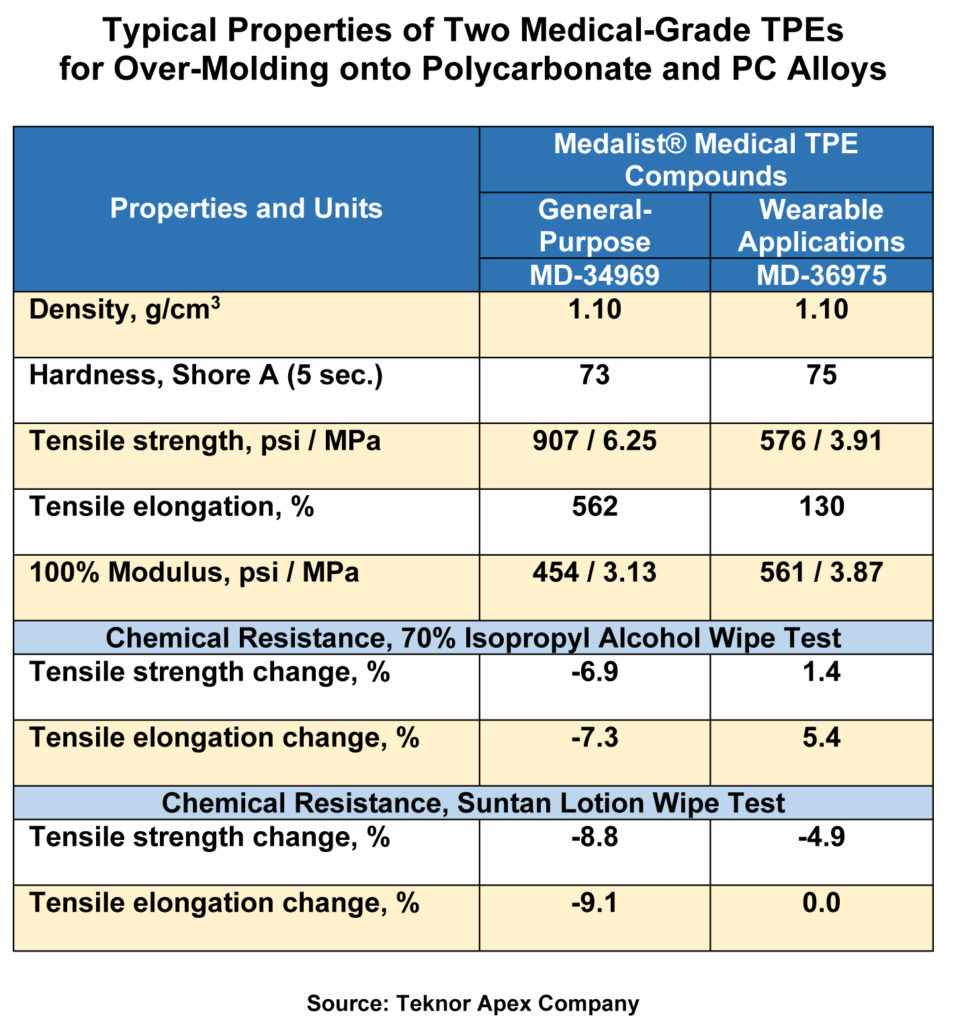
Teknor Apex has developed a new series of Medalist medical-grade thermoplastic elastomer compounds for overmoulding exhibit strong adhesion to medical grade polycarbonate (PC) and PC alloys and include formulations with enhanced resistance to chemicals encountered in wearable applications.
Medalist MD-34900 series compounds, available in 50, 60, and 70 Shore A grades, are for general medical overmoulding applications, while the MD-36975 grade is a 75 Shore A TPE designed specifically for overmoulding in wearable devices. The company said that the compound has an excellent resistance to the lotions and disinfectants that are typically encountered in this application.

Project with Covestro
In a joint project with Covestro LLC, these materials were overmoulded onto various medical grades of Makrolon PC as well as Bayblend and Makroblend PC alloys, using both insert and multi-shot moulding. The project included tests to evaluate adhesion, processability, and chemical resistance. Peel strength data showed the Medalist compounds exhibiting excellent adhesion to Covestro’s engineering thermoplastics.
Teknor Apex recommends the new compounds for wearable devices, where TPEs contribute comfort; ‘soft-touch’ or cosmetic grips for medical device housings, handheld devices, and instruments; and seals and gaskets.
“Medalist TPEs are excellent alternatives to liquid silicones used in overmoulding because they do not require the use of a primer, have a shorter cycle time (avoiding the lengthy curing process), and can be processed on conventional injection moulding equipment,” said Ross van Royen, Senior Market Manager for Teknor Apex.
Competence in overmoulding
The new compounds for the medical device industry are an outgrowth of work carried on by Teknor Apex to develop overmoulding TPE formulations for consumer products.
“We’ve made substantial investments in adhesion-modified technologies for TPEs, increasing our understanding of adhesion, developing more cost-effective formulations, and improving the bonding capabilities of these products in complex part designs,” said van Royen.
The company has launched an online resource for overmolding to share their insights:
https://www.teknorapex.com/overmolding-academy
The MD-34900 series and MD-36975 compounds are available worldwide. As medical grade TPEs, they are subject to strict formulation controls, are made only with FDA-listed food grade ingredients, are ISO 10993-5 compliant for biocompatibility, and are compliant with CONEG, RoHS, and California Proposition 65 requirements. Teknor Apex produces these compounds in several ISO-13485-certified facilities, ensuring security of supply.
Teknor Apex will introduce the new Medalist TPEs at the world’s largest medical design and manufacturing event MD&M West (11-13 February 2020, Anaheim, CA, USA). And together with project partner Covestro it will present a white paper with details of the experiments and product performance.
Teknor Apex at MD&M West, stand 2121
Covestro at MD&M West, stand 2221
