Ineos Styrolution has introduced a new Styroflex grade for medical applications. The company said that the new grade provides an attractive mix of properties making it ideal for various medical tubing applications – ranging from use in traditional IV sets to more complex multi-layer tubing structures. Key material properties include good bonding performance, good kink resistance and clarity, and the ability to be processed on standard tubing extrusion equipment at superior processing rates when compared to other materials. This combination of properties makes Styroflex 4G80 an attractive option for tubing applications that historically have been developed with different polymers, Ineos said.
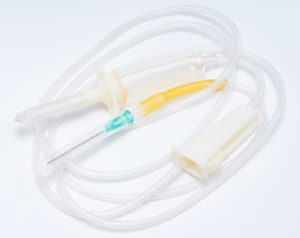
Alexander Silvestre, Global Director, Healthcare, said: “Styroflex 4G80 has been developed in close cooperation with some of our partners to ensure a market need was properly met. It was designed to meet the demanding needs for medical tubing, but also cater to the desires for some medical OEMs looking for an alternate solution to what is currently offered.”
Styroflex 4G80 is available with Ineos Styrolution’s Essential HD Package (risk class 1 & 2) which includes up to twelve months notification of change (NOC) with a signed long-term supply contract, locked formulations as defined in a Drug Master File (DMF) which will be filed soon with the FDA, and a variety of regulatory compliance documents and biocompatibility information (e.g. USP Class VI, ISO 10993, food contact statements).
The company introduced the material at the MD&M West in Anaheim, CA; USA, last week.
