Manufacturers of medical devices deal intensively with the materials to be used before the development phase. In the field of plastics, PVC has proven itself for many medical applications. As for infusion systems each system comprises a pin, a drip chamber, a transparent infusion line, a flow controller and a connector. This material is increasingly subject to criticism, e.g. because of problems with plasticizers. With serious possible consequences: PVC plasticizers can be released. Certain active ingredients can also form deposits on the surface of the PVC material which impairs the effect of the respective drug. Medical products that are produced in very large numbers must, on the one hand, be economical to manufacture despite the highest demands on product safety and hygiene. On the other hand, the requirements are constantly increasing to ensure the health of staff and patients.
In an effort to comply with this requirement profile, there is a growing tendency to rely on alternative materials instead of PVC. On the one hand, not all alternative plasticizers have been investigated and documented as extensively as is the case with DEHP; on the other hand, this by no means solves the environmental problem that arises when disposing of PVC. In comparison, TPE perform well because they are completely recyclable. Neither PVC, nor thermosetting plastics nor latex can be recycled simply or completely. In addition, PVC and thermosetting plastics generate large amounts of waste during production and processing, and plasticizers can be released from PVC. Even during the production of the granulate, the energy consumption is lower than with soft PVC. With TPE granules, the potential for the migration of undesirable substances is minimized.
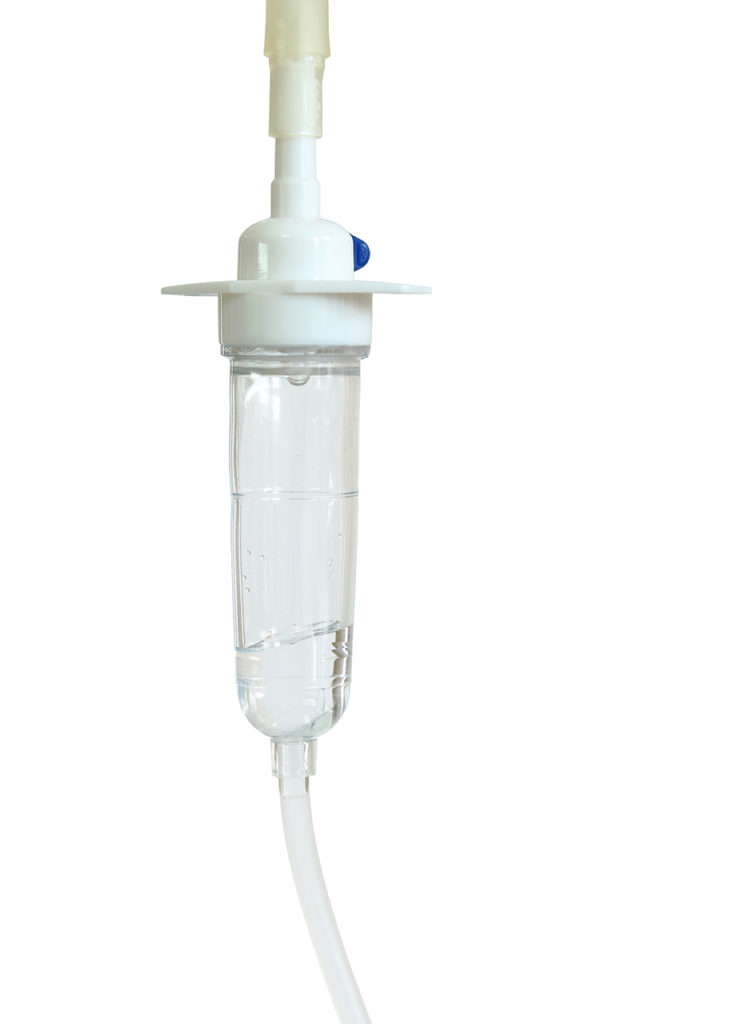
Actega DS supplies ProvaMed TPE D1341, D1345 and D1349 TP, especially designed for the manufacture of drip chambers. These grades represent a safe solution for the high demands on performance and safety of medical products and permit the implementation of complex constructions in elastic and transparent products, the company said.
Apart from absolute harmlessness for health, drip chambers must feature outstanding transparency accompanied by a good balance of flexibility and rigidity. Transparency is one of the key words for guaranteeing impeccable optical control of the drip process as well as swift and easy adjustment of the fluid level. Provamed formulae have been formulated accordingly, with all variants ultra-transparent. The grades can be sterilized with standard processes such as ethylene oxide and gamma rays without impairing the material properties or bonding functions between the drip chamber and the tube.
Actega DS points out that a range of regulatory requirements needs to be met by TPE for drip chambers. According to USP Class VI, biological testing and biological reactivity tests need to be conducted. The biocompatibility of TPE formulae in accordance with ISO 10993 must be confirmed, whereby in vitro tests concern the cytotoxicity and viability of cell cultures. Production must be confirmed in line with GMP Directive (EC) 2023/2006 and conformity with EC 1935/2004 and EU 1072011 as well as various FDA chapters for contact with food. The materials must also be free of heavy metals. All variants of TPE for drip chambers comply with these regulations. By using the documented and ISO 10993-5 certified TPE, a stress-free and smooth qualification of the medical devices is possible.
